What Relationship Does Avogadros Law Describe
The link between the amount of gas n and the volume V is investigated via Avogadros law v. Avogadros Law is stated mathematically as follows.
What does it mean that mass and moles are proportionally related.

. Avogadros Law is the relation which states that at the same temperature and pressure equal volumes of all gases contain the same number of molecules. The Avogadro law is. What relationship does Avogadros law describe.
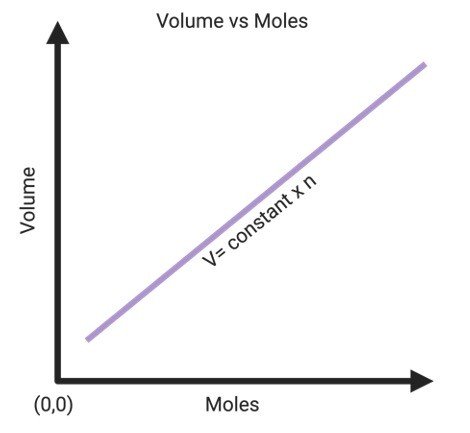
A modern statement is. Its a direct relationship meaning the volume of a gas is directly propotional to the number of moles the gas sample present. Its a direct relationship which means the volume of a gas is proportional to the number of moles contained in the gas sample.
Avogadros law states that equal volumes of all gases at the same temperature and pressure have the same number of molecules For a given mass of an ideal gas the volume and amount moles of the gas are directly proportional if the temperature and pressure are constant. Hence the volume of a gas at constant pressure and temperature is directly proportional to the number of moles. Relationship between volume and number of moles of a gas at constant temperature and pressure.
Avogadros law a statement that under the same conditions of temperature and pressure equal volumes of different gases contain an equal number of molecules. The Avogadro law is. The law is important because helps us save time and money in the long-run24 мая 2015 г.
Avogadros law investigates the relationship between the amount of gas n and volume v. The law is significant because it allows us to save time and money over time. The Avogadro law is.
Avogadros law states that equal volumes of all gases at the same temperature and pressure have the same number of molecules For a given mass of an ideal gas the volume and amount moles of the gas are directly proportional if the temperature and pressure are constant. This empirical relation can be derived from the kinetic theory of gases under the assumption of a perfect ideal gas. Avogadros constant 602x10²³ 1 mole of gas occupies 224dm³ at stp prepare 4-phenyl-2-butanone from acetoacetic ester Previous.
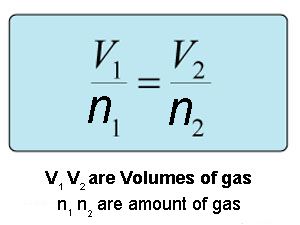
V n k V n k where V is the volume of the gas n is the number of moles of the gas and k is a proportionality constant. Equal volumes of gases at the same temperature and pressure have an equal number of molecules. The number of molecules or atoms in a specific volume of ideal gas is independent of size or the gas molar mass.
What does Daltons law state. The law is approximately valid for real gases at sufficiently low pressures and high temperatures. Volume ratios must be related to the relative numbers of molecules that react.
What relationship does Avogadros law describe. Equal volumes of gases at the same temperature and pressure have an equal number of molecules. A mathematical expression that summarizes Avogadros law is.
The relationship between pressure and volume. Amedo Avogadro found the relationship between the volume of a gas and the number of molecules contained in the volume. V n PT constant.
Avogadros law sometimes referred to as Avogadros hypothesis or Avogadros principle or Avogadro-Ampères hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The relationship between temperature and volume B. What relationship does Avogadros law describe.
Equal volumes of gases at the same temperature and pressure have an equal number of molecules. What relationship does Avogadros law describe. What relationship does Charless law describe.
The law states that equal volume of all gases at the same temperature and pressure contains the same number of molecules or moles. In chemistry and physics Daltons law also called Daltons law of partial pressures states that in a mixture of non-reacting gases the total pressure exerted is equal to the sum of the partial. The relationship between moles and temperature C.
According to Avogadros Hypothesis other conditions remaining identical equal volumes of all gases will contain same no.

Avogadro S Law Statement Formula Derivation Solved Examples Of Avogadro S Law

Avogadro S Law With Graphs And Applications Chemistrygod

Avogadro S Law Animation Youtube

Avogadro S Law Equal Volumes Of Different Gases At The Same Temperature And Pressure Have The Same Number Of Moles Example Cl2 G H2 G Ppt Video Online Download

Avogadro S Law Statement Formula Derivation Solved Examples Of Avogadro S Law

Avogadro S Law Definition Formula Examples Avogadro S Law Science Notes Chemistry Classroom
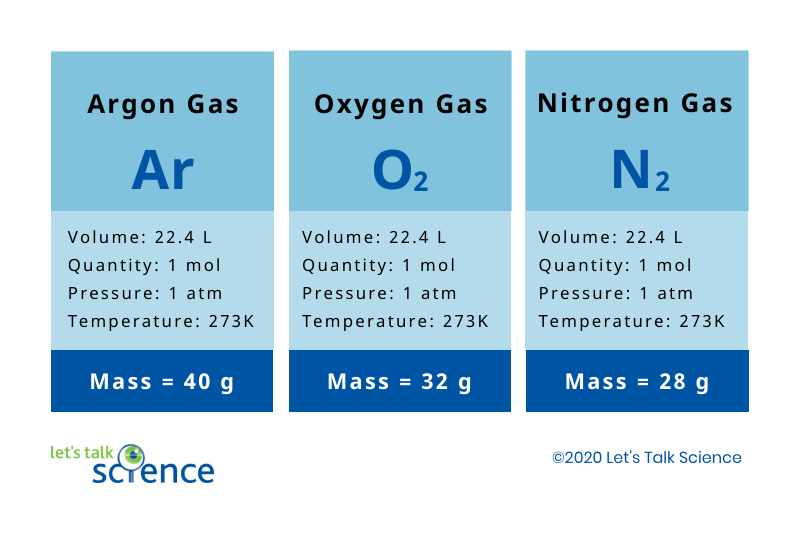
Avogadro And The Ideal Gas Law Let S Talk Science
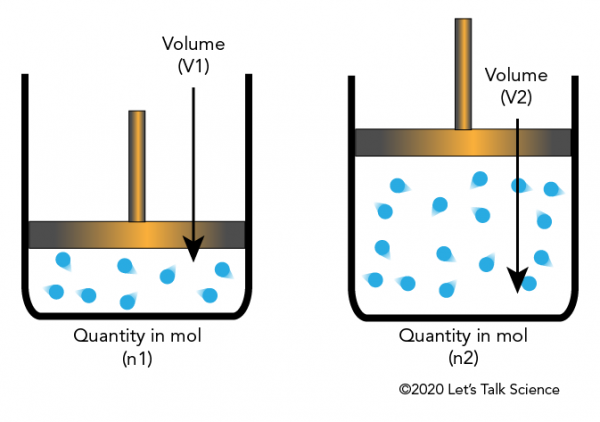
Avogadro And The Ideal Gas Law Let S Talk Science

Avogadro S Law With Graphs And Applications Chemistrygod

Avogadro S Law Practice Problems Youtube

Icse Grade 10 Chemistry Mole Concept And Stoichiometry Lessons Exercises And Practice Tests Chemistry Mole Concept Lesson
Avogadro S Law Formula Calculation Definition And Examples

Avogadro S Law With Graphs And Applications Chemistrygod

Avogadro S Law Statement Formula Derivation Solved Examples Of Avogadro S Law

Avogadros Law Avogadro S Law Chemistry Classroom Thermodynamics



Comments
Post a Comment